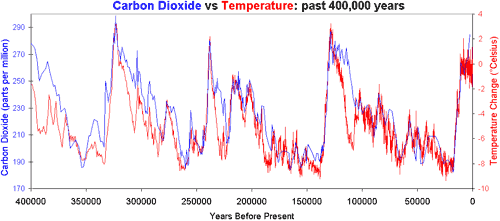
Proponents of anthropogenic global warming insist that man's use of fossil fuels is dangerously warming the world. But is causing B, or B causing A? This graph shows CO2 and temperature changes over the last 400,000 years:
You will notice that the temperature sometimes rises before the CO2 levels. Why? As this website at Columbia explains:
The primary source of carbon/CO2 is outgassing from the Earth's interior at midocean ridges, hotspot volcanoes, and subduction-related volcanic arcs. Much of the CO2 released at subduction zones is derived from the metamorphism of carbonate rocks subducting with the ocean crust. Much of the overall outgassing CO2, expecially as midocean ridges and hotpot volcanoes, was stored in the mantle when the Earth formed. Some of the outgassed carbon remains as CO2 in the atmosphere, some is dissolved in the oceans, some carbon is held as biomass in living or dead and decaying organisms, and some is bound in carbonate rocks. Carbon is removed into long term storage by burial of sedimentary strata, especially coal and black shales that store organic carbon from undecayed biomass and carbonate rocks like limestone (calcium carbonate).
How much is tied up in carbonate rocks? I believe that I have read that there is 40x more CO2 in carbonate rocks than in the atmosphere. If that seems unlikely, think of all the limestone visible on the surface, under the ocean where it is being subducted, as well as chalk, also a carbonate. From Cal State Los Angeles:
Limestone, a sedimentary rock which contains silica, and composed largely of the mineral calcite. It is also 10% of the total volume of all sedimentary rocks, and many marine organisms live on it. Limestone, CaCO2, is a type of sedimentary rock making up 10% of all sedimentary rocks on earth.
CO2 is also dissolved in seawater, a lot of it:
Of the three places where carbon is stored—atmosphere, oceans, and land biosphere—approximately 93 percent of the CO 2 is found in the oceans. The atmosphere, at about 750 petagrams of carbon (a petagram [Pg] is 10 15 grams), has the smallest amount of carbon.
So rising temperatures warms up seawater and:
Solubility of a gas in water tends to decrease with increasing temperature, and solubility of a gas in an organic solvent tends to increase with increasing temperature.
So temperatures rise, CO2 comes out of the oceans and ends up in the atmosphere. A warming world will have more CO2 in the atmosphere, but rising temperatures is likely the cause not the result.
No comments:
Post a Comment